Innovation is in our DNA
Custom cell line development solutions for complex biologics.
Innovation is in our DNA
Custom manufacturing solutions for complex biologics.
Introducing Omnicho™
the first high performance cho-k1 transient kit

Keep your programs moving forward
Despite the continued advances in human health through the development of biologics to enable treatment of complex diseases, protein expression and manufacturing solutions continue to pose a significant hurdle to clinical and preclinical development of complex biologics and challenging antibodies. Celltheon’s experienced team leverages decades of combined experience and a leading edge suite of protein expression technologies to provide custom solutions for your products at all phases of development.
Centralized production, purification, and analytical services.
Developability
OPTIMIZATION

Cell line development &
Process development
Analytical
characterization
GLP bioproduction
and scale up
Celltheon Technologies
-
OmniCHO Transient Expression™First high titer CHO-K1 based transient expression system
-
pCT™ Vectors Genetic elements for homogenous, high expressing clones (CT STABILIZER™ & SIREN Promoter™)
-
SUPERCELL™ Metabolic & protein folding engineered CHO cells. Custom KO/KI solutions
-
GOLDILOCKS TRANSPOSASE™ High Capacity, High Efficiency, High Titer
-
CHROMASelect™ Next gen platform screening >100M clones for mannose, sialylation, titer, aggregation, and clipping of mAbs and non-mAbs
-
SMART BIOPROCESSING™ OTS & Custom bioprocessing solutions to improve solubility, titer, PTMs, truncation
Lorem ipsum dolor sit amet, consectetur adipisicing elit. Optio, neque qui velit. Magni dolorum quidem ipsam eligendi, totam, facilis laudantium cum accusamus ullam voluptatibus commodi numquam, error, est. Ea, consequatur.
CELLTHEON Smart™ Expression Platform
A Universal Platform for all protein formats
Celltheon has predefined preclinical development workflows for various protein formats. Please see our Development Blueprint Brochure



fusion (type 1)


The Bridge from Drug Discovery to Manufacturing
Transient Production
Transient Production
Celltheon’s experts enable rapid production, purification and characterization of hundreds of protein variants to enable seamless transition into lead candidate selection.
- Average mAb titers of 2.0gm/L in 4-5 day culture
- High quality analytical characterization (Kinetics, SE-HPLC, LAL endotoxin, etc.)
- Small to large scale production of hundreds of variants in CHO and HEK293 cells
- Purified, low endotoxin proteins using any chromatographic method for in vitro and in vivo studies
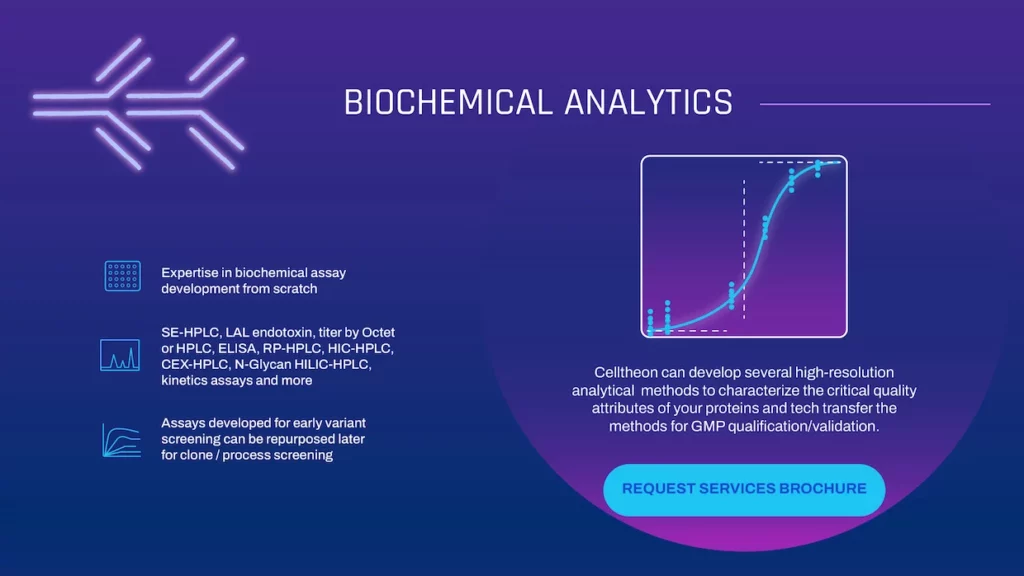
Biochemical Analytics
BIOCHEMICAL ANALYTICS
Celltheon can develop several high-resolution analytical methods to characterize the critical quality attributes of your proteins and tech transfer the methods for GMP qualification/validation.
- Expertise in biochemical assay development from scratch
- SE-HPLC, LAL endotoxin, titer by Octet or HPLC, ELISA, RP-HPLC, HIC-HPLC, CEX-HPLC, N-Glycan HILIC-HPLC, kinetics assays and more
- Assays developed for early variant screening can be repurposed later for clone / process screening
Biological Activity Assay Dev
BIOLOGICAL ACTIVITY
ASSAY DEVELOPMENT
Using our expertise in cell biology, our team can provide critical cell-based assay development services to thoroughly characterize the biological activity of your protein.
- Cell based assay method development and method transfer for qualification and validation for product release
- Enables complete characterization of variants during discovery and clone / process characterization during IND-enabling development phases
- Various assays including: FACS, cytokine release, cell killing assays, ADCC, CDC, transwell, and custom development
Tox Material Production
TOX MATERIAL PRODUCTION
The CELLTHEON SMART™ platform enables rapid production of high quality material for tox studies. Celltheon has stirred-tank bioreactors and purification systems to produce up to 100 grams / production batch.
- High concentration formulation available with compatible proteins. Rapid pre-formulation development services available for incompatible proteins.
- Celltheon’s high titer stable pools and clones enable production of 10s-100s of grams within 3-4 weeks
- Complete COA with product quality characterization provided upon release
- Rigorous CIP and maintenance of equipment enables low endotoxin material production (<0.05EU/mg)
Cell Line Development
CELL LINE DEVELOPMENT
mAbs are expressed in the CELLTHEON SMART™ platform simply & quickly at 5-10g/L titers and DEP’s are developed equally rapidly with generally equally high titers using the CELLTHEON SUPERCELL™ cell line, or through custom development programs with a focus on deep biology to address the unique challenges associated with each protein.
- High titers of >8g/L clones and >3g/L pools and stable clones >60 generations
- Rapid tech transfer timelines: 4.5 months to RCB or 6 months with upstream/downstream PD
- Clone selection based on clone performance and CQAs
- Suite of technologies including engineered promoters, genetic elements, engineered cells, novel transposases for maximized titers
DSPD
DOWNSTREAM PROCESS DEVELOPMENT
Our downstream process development team can develop a scalable 3-4 column chromatography process that is optimized for high product yield and purity, validated using various analytical methods.
- Develop scalable, manufacturing-ready chromatography processes with high yield/purity
- Screen multiple viral inactivation and filtration procedures to ensure product solubility and stability
- Clarification process development via depth filtration and formulation via TFF
- Expertise with nearly all chromatography methods and resin types (IEX, HIC, Affinity, IMAC, SEC, etc.)
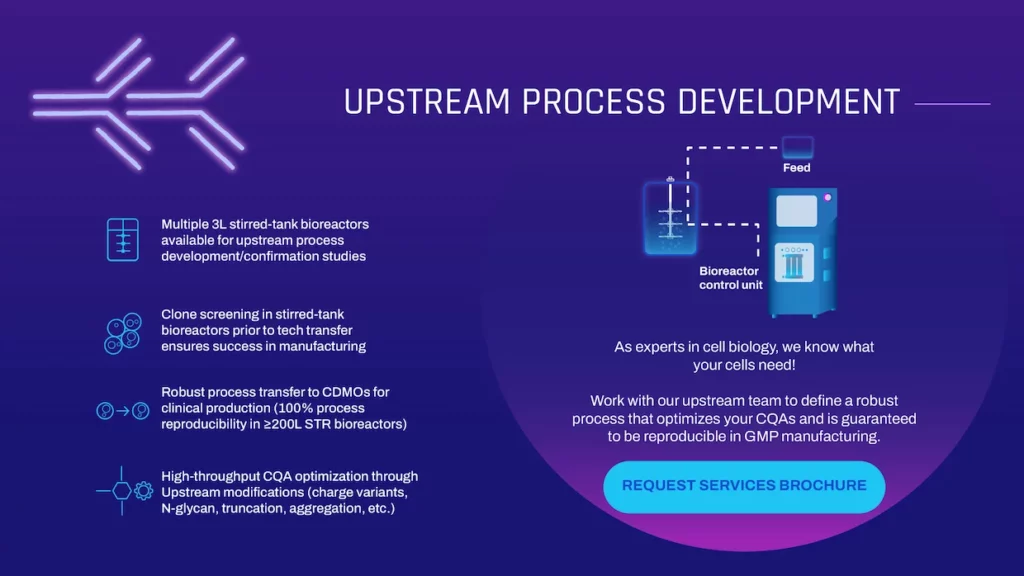
UPSTREAM PROCESS DEVELOPMENT
As experts in cell biology, we know what your cells need! Work with our upstream team to define a robust process that optimizes your CQAs and is guaranteed to be reproducible in GMP manufacturing.
- Multiple 3L stirred-tank bioreactors available for upstream process development/confirmation studies
- Clone screening in stirred-tank bioreactors prior to tech transfer ensures success in manufacturing
- Robust process transfer to CDMOs for clinical production (100% process reproducibility in ≥200L STR bioreactors)
- High-throughput CQA optimization through Upstream modifications (charge variants, N-glycan, truncation, aggregation, etc.)
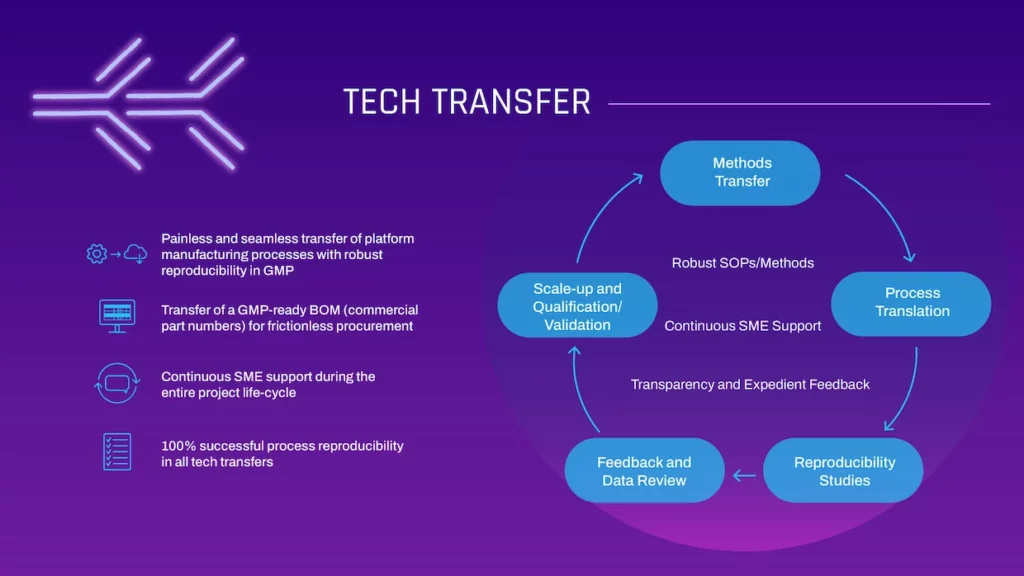
TECH TRANSFER
- Painless and seamless transfer of platform manufacturing processes with robust reproducibility in GMP
- Transfer of a GMP-ready BOM (commercial part numbers) for frictionless procurement
- Continuous SME support during the entire project life-cycle
- 100% successful process reproducibility in all tech transfers
- Methods Transfer
- Process Translation
- Reproducibility Studies
- Feedback and Data Review
- Scale-up and Qualification/ Validation
- Robust SOPs/Methods
- Continuous SME Support
- Transparency and Expedient Feedback
Capabilities that shorten your preclinical development timelines
A Track Record for Success

Total proteins delivered

Difficult to express proteins (DEPs)

Tech transferred cell lines/ Processes in GMP

Customers served




Total proteins delivered
Difficult to express proteins (DEPs)
Tech transferred cell lines/ Processes in GMP
Customers served
About Celltheon
MEET OUR TEAM
Celltheon develops leading edge technologies and innovative solutions for the next generation of biotherapeutics. Celltheon offers platformed and custom end-to-end solutions for drug developability, cell line development, and process development to its clients, to bridge the gap between early drug development and manufacturing.
Celltheon has a comprehensive suite of patented industry leading technologies and know-how to provide end-to-end solutions as well as custom point solutions. Located in the SF Bay Area, Celltheon has worked on 1000+ proteins for its 100+ global clients with a 100% track record of success. We do not workaround – we innovate, and we deliver!